Table of Contents
Custom Morpholinos
Morpholino Antisense Oligos function by binding to pre-messenger or messenger RNA, blocking access to the RNA. Morpholinos function solely by a steric block mechanism, so they do not require or use RNase H for their activity. When properly selected and targeted, Morpholinos generally afford equal or better efficacy and greatly superior specificity than any other antisense structural type. However, inappropriately selected or targeted Morpholinos will have little or no activity. Accordingly, Gene Tools provides an Oligo Design Service at no added cost. Gene Tools follows our Targeting Guidlines to select appropriate oligo sequences suitable for inhibiting the activity of the genes of your choice.
For quantity and price information on custom-sequence Morpholino antisense oligos go to Current Price List.
For selecting and ordering Morpholino oligos visit our Online Store.

Control Oligos
Standard Control: A Morpholino Standard Control oligo is offered at a reduced price per nanomole for small orders (100 to 300 nanomoles) to minimize the expense of antisense experiments. This Standard Control oligo is a 25-mer having the sequence:
5'-CCT CTT ACC TCA GTT ACA ATT TAT A 3'
This oligo should have no target and very little biological activity - except in reticulocytes from thallasemic humans having a splice-generating mutation at position 705 in beta-globin pre-mRNA. In those cells this oligo will correct a splicing error and thereby generate a correctly-spliced mRNA which codes for normal beta-globin chains. However, slight changes from mock-injected embryos have been observed in zebrafish; any single sequence is likely to have some effect on gene expression. The Standard Control is a widely used and often reported sequence that is a pretty good, but not perfect, negative control.
For quantity and price information on this Standard Control oligo go to Current Price List.
Random Control: The Random Control 25-N is a mixture of many oligo sequences, made be adding a mixture of activated Morpholino subunits at each step of the synthesis cycle. There are in theory 4^25 different sequences in a large batch of the random control mixture. In a solution containing 1 mM of the Morpholino backbone, any one sequence is present in vanishingly small concentration.
Custom Negative Controls: Custom-sequence Morpholino negative control oligos can also be ordered. If the standard or random control oligos are unsuitable as negative controls for your experiments, we recommend using the invert of your antisense sequence as your negative control. This invert-antisense has the advantage of having the same length and base composition as your antisense oligo.
Here is an example based on an arbitrary antisense sequence:
antisense sequence: 5'-AAC GAA CGA ACG AAC GAA CGA ACG C
invert antisense: 5'-CGC AAG CAA GCA AGC AAG CAA GCA A
A less desirable negative control is the sense sequence. Sometimes odd expression modulation is observed with sense controls, such as upregulation of the gene of interest (hypothetically due to DNA interactions).
antisense sequence: 5'-AAC GAA CGA ACG AAC GAA CGA ACG C
sense sequence: 5'-GCG TTC GTT CGT TCG TTC GTT CGT T
Custom Specificity Controls: Custom-sequence Morpholino specificity control oligos include non-overlapping antisense control oligos and five-mispair control oligos.
For rigorous specificity studies we recommend using a second non-overlapping oligo targeting the same RNA as the first antisense oligo. If the outcome of a knockdown is recapitulated when using a second oligo targeting the same mRNA as the first, this supports the hypothesis that the outcome observed was due to interaction with the targeted RNA and not an unexpected RNA.
Another specificity control is the five-mispair oligo, an option used for antisense studies throughout the early history of antisense; we recommend that you do not use the five mispair oligo as a specificity control. Unexpected effects sometimes appear when using five-mispair oligos. To most effectively decrease interaction with the antisense oligo's target RNA, the mispairs should be distributed through the sequence of the five-mispair control oligo.
antisense sequence: 5'-AAC GAA CGA ACG AAC GAA CGA ACG C
5-mispair control: 5'-AAg GAA gGA ACc AAC GAA CcA AgG C
It is noteworthy that in early studies of sequence specificity utilizing such mispaired controls, Morpholino oligos were found to afford high-specificity target inhibition over a concentration range several hundred fold greater than that afforded by corresponding Phosphorothioate oligos (Summerton et al., 1997; Summerton & Weller, 1997a).
For quantity and price information on custom-sequence Morpholino control oligos go to Current Price List.
End Modifications
Gene Tools can provide Morpholino oligos with modifications on the 3' and/or 5' ends of the oligos. Available structures are shown below.
Linked index of end modifications:
3'-Fluorescent tags and quencher
- 3'-Carboxyfluorescein (Now available as a 5' modification for Vivo Morpholinos; See here for description)
- 3'-Gene Tools Blue
- 3'-Lissamine
- 3'-Dabcyl
- 3'-Sulfo-Cyanine5
- 3'-AF488
3'-Affinity tag and functional groups for chemical linkage
- 3'-Biotin synthesized prior to 24 Jun 2016
- 3'-Biotin synthesized 24 Jun 2016 and after
- 3'-Primary Amine
- 3'-Disulfide Amide
- 3'-Pyridyl dithio
- 3'-Puromycin
- 3'-Cholesterol
5'-end Modifications
Modifications for Click Chemistry
- 3'-Azide
- 3'-Alkyne
- 3'-Cyclooctyne v1, no longer available
- 3'-Cyclooctyne v2, no longer available
- 3'-Cyclooctyne v3, current version
- 5'-Azide (Now available as a 5' modification for Vivo Morpholinos; See here for description)
- 5'-Alkyne
- 5'-Cyclooctyne v1, no longer available
- 5'-Cyclooctyne v2, no longer available
- 5'-Cyclooctyne v3, current version
3'-Fluorescent tags and quencher
3'-Carboxyfluorescein
Green-emitting fluorescent tag
Adds 489 Daltons to oligo mass
Production number suffix:-F
Example: 12-04Apr05A-F Excitation peak: 501.5 nm
Emission peak: 524.5 nm
 Back to Index of Modifications
Back to Index of Modifications3'-Gene Tools Blue*
Blue-emitting fluorescent tag
Adds 750 Daltons to oligo mass
Production number suffix: GB
Example 12-04Apr05A-GB Excitation peak: 433 nm
Emission peak: 465 nm *Patent pending

The following filters and dichroic mirror from Chroma Technology Corp. match the excitation and emission of Gene Tools Blue.
AT440DC beamsplitter
ET405/40x excitation
ET460/36m emission
3'-Lissamine
Red-emitting fluorescent tag
Adds 784 Daltons to oligo mass
Production number suffix:-L
Example 13-04Apr05A-L Excitation peak: 575.0 nm
Emission peak: 593.0 nm
 LISSAMINE NOTE: Lissamine (sulforhodamine B) can decrease the aqueous solubility of many Morpholino sequences. If you need a fluorescent tag and a green fluor will suffice, we suggest you have your oligos labelled with carboxyfluorescein. If you must use a lissamine tag, be sure that after you add water you heat and aggressively vortex the oligo solution until it is completely dissolved. Do not attempt to make a stock solution of a lissaminated oligo with a concentration greater than 1 mM; lower concentrations, such as 500 uM, are a better choice for these oligos.
Back to Index of Modifications
LISSAMINE NOTE: Lissamine (sulforhodamine B) can decrease the aqueous solubility of many Morpholino sequences. If you need a fluorescent tag and a green fluor will suffice, we suggest you have your oligos labelled with carboxyfluorescein. If you must use a lissamine tag, be sure that after you add water you heat and aggressively vortex the oligo solution until it is completely dissolved. Do not attempt to make a stock solution of a lissaminated oligo with a concentration greater than 1 mM; lower concentrations, such as 500 uM, are a better choice for these oligos.
Back to Index of Modifications3'-Dabcyl
Fluorescence quencher
Adds 382 Daltons to oligo mass
Production number suffix: -Q
Example 14-04Apr05A-Q
 Back to Index of Modifications
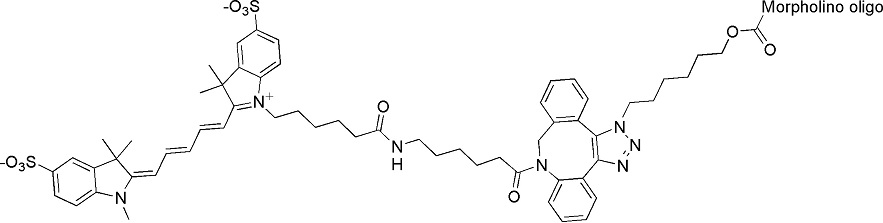
Back to Index of Modifications3'-Sulfo-Cyanine5
A red, water soluble fluorophore
Adds 1111 Daltons to oligo mass
Production number suffix: -RZ
Example: 15-08Apr24A-RZ Excitation peak: 646 nm
Emission peak: 662 nm
Quantum Yield: 0.28
Extinction Coefficient: 271000 L/mol/cm
 Back to Index of Modifications
Back to Index of Modifications3'-AF488
A bright photostable hydrophilic fluorophore that emits in the green channel.
Adds: 1003 Daltons to mass
Production number suffix: -GZ
Example: 15-08Apr24A-GZ Excitation Max: 495 nm
Emission Maximum: 519 nm
Quantum Yield: 0.91
Extinction Coefficient: 73000
 Back to Index of Modifications
Back to Index of Modifications
3'-Affinity tag and functional groups for chemical linkage
3'-Biotin v1
Affinity tag for avidin or streptavidin, no longer available: see 3'-Biotin v2 below for our improved version.
This structure is on biotinylated oligos synthesized prior to 24 Jun 2016
Adds 393 Daltons to oligo mass
Production number suffix:-B
Example 16-04Apr05A-B
 Back to Index of Modifications
Back to Index of Modifications3'-Biotin v2
Affinity tag for avidin or streptavidin, for syntheses after 24 Jun 2016 (now available)
Adds 357 Daltons to oligo mass
Production number suffix:-B
Example 16-06Jul16A-B
 Back to Index of Modifications
Back to Index of Modifications3'-Primary Amine
Functional group for linking a compound to a Morpholino oligo.
Adds 71 Daltons to oligo mass
Example 15-04Apr05-A
 Back to Index of Modifications
Back to Index of Modifications3'-Disulfide Amide
Functional group for linking a compound to a Morpholino oligo.
Can be reduced to a thiol. Not activated for substitution; more stable than activated disulfide.
Adds 219 Daltons to oligo mass
Production number suffix:-S
Example 12-04Apr05A-S
 Back to Index of Modifications
Back to Index of Modifications3'-Pyridyl dithio
Functional group for linking a compound to a Morpholino oligo.
Activated for substitution; less stable than disulfide amide.
Adds 352 Daltons to oligo mass
Production number suffix: -PD
Example 12-04Apr05A-PD
 3'-PYRIDYLDITHIO NOTE: This group is degraded by high temperatures, therefore we do not sterilize Morpholino oligos with the 3'-pyridyldithio modification. These oligos are shipped as non-sterile lyophilized solids. After conjugation with another moiety, the product might be stable to heat so that it can be sterilized by autoclaving; otherwise, the oligos can likely be sterilized by filtration though a polysulfone membrane filter if necessary.
3'-PYRIDYLDITHIO NOTE: This group is degraded by high temperatures, therefore we do not sterilize Morpholino oligos with the 3'-pyridyldithio modification. These oligos are shipped as non-sterile lyophilized solids. After conjugation with another moiety, the product might be stable to heat so that it can be sterilized by autoclaving; otherwise, the oligos can likely be sterilized by filtration though a polysulfone membrane filter if necessary.Back to Index of Modifications
3'-Puromycin
This end modification is not on the store, and available only on request.
Adds 672 Daltons to oligo mass
 Back to Index of Modifications
Back to Index of Modifications3'-Cholesterol
The cholesterol conjugate of oligonucleotide can enhance passage through lipid membranes and uptake into cells and tissue.
Adds 543 Daltons to oligo mass
Production number suffix:-X
Example 12-24Apr05A-X
 Back to Index of Modifications
Back to Index of Modifications
5'-end Modifications
5'-Primary Amine
Functional group for linking a compound to a Morpholino oligo.
Adds 266 Daltons to oligo mass (plus additional 42 Daltons*)
* If the 3'-end is not otherwise substituted then the terminal morpholine nitrogen is capped with a 3'-acetyl, adding an additional 42 Daltons to the oligo mass.
Production number suffix: -5A
Example 12-04Apr05A-5A
 Back to Index of Modifications
Back to Index of Modifications5'-Dabcyl
Fluorescence quencher
Adds 517 Daltons to oligo mass
Production number suffix: -5Q
Example 12-04Apr05A-5Q
 Back to Index of Modifications
Back to Index of Modifications5'-Triethylene Glycol
Inert oligo end
Adds 174 Daltons to oligo mass
Production number suffix: -5T
Example 12-04Apr05A-5T
 Back to Index of Modifications
Back to Index of Modifications5'-Sulfo-CY5
This is a red, water soluble fluorophore.
Adds 1246 Daltons to oligo mass
 Back to Index of Modifications
Back to Index of Modifications5'-AF488
A bright photostable hydrophilic fluorophore that emits in the green channel.
Adds 1138 Daltons to oligo mass
 Back to Index of Modifications
Back to Index of Modifications
Modifications for Click Chemistry
3'-Azide
Functional group for click chemistry linking of a compound to a Morpholino oligo.
Adds 169 Daltons to oligo mass
Production number suffix: -Z
Example 12-04Apr05A-Z
 Note that this is suitable for copper-catalyzed click chemistry or for addition to constrained cyclic alkynes for copper-free click chemistry.
Back to Index of Modifications
Note that this is suitable for copper-catalyzed click chemistry or for addition to constrained cyclic alkynes for copper-free click chemistry.
Back to Index of Modifications3'-Alkyne
Functional group for click chemistry linking of a compound to a Morpholino oligo.
Adds 110 Daltons to oligo mass
Production number suffix: -E
Example 12-04Apr05A-E
 Back to Index of Modifications
Back to Index of Modifications3'-Cyclooctyne v1, no longer available
3'-Cyclooctyne v1 is a functional group for linking a compound to a Morpholino oligo using copper-free click chemistry .
This structure is in 3'-cyclooctynylated oligos synthesized prior to 12 Aug 2021 and is no longer available: see 3'-Cyclooctyne v3 below for our improved version.
Adds 222 Daltons to oligo mass
Production number suffix: -C
Example 12-04Apr05A-C
 Back to Index of Modifications
Back to Index of Modifications3'-Cyclooctyne v2, no longer available
3'-Cyclooctyne v2 is a functional group for linking a compound to a Morpholino oligo using copper-free click chemistry.
This structure is in 3'-cyclooctynylated oligos synthesized from 12 Aug 2021 through 31 Dec 2021 and is no longer available: see 3'-Cyclooctyne v3 below for our improved version.
Adds 238 Daltons to oligo mass
Production number suffix: -C
Example 12-04Sep21A-C
 Back to Index of Modifications
Back to Index of Modifications3'-Cyclooctyne v3, current version
3'-Cyclooctyne v3 is a functional group for linking a compound to a Morpholino oligo using copper-free click chemistry.
This structure is in cyclooctynylated oligos synthesized after 31 Dec 2021: current version.
Adds 178 Daltons to oligo mass
Production number suffix: -C
Example 03-10Jan22A-C
 The new (v3) cyclooctynes have faster kinetics (more than a tenfold rate increase) relative to the prior versions.
The new (v3) cyclooctynes have faster kinetics (more than a tenfold rate increase) relative to the prior versions.
Back to Index of Modifications
5'-Azide
Functional group for click chemistry linking of a compound to a Morpholino oligo.
Adds 304 Daltons to oligo mass
Production number suffix: -5Z
Example 12-04Apr05A-5Z
 Note that this is suitable for copper-catalyzed click chemistry or for addition to constrained cyclic alkynes for copper-free click chemistry.
Back to Index of Modifications
Note that this is suitable for copper-catalyzed click chemistry or for addition to constrained cyclic alkynes for copper-free click chemistry.
Back to Index of Modifications5'-Alkyne
Functional group for click chemistry linking of a compound to a Morpholino oligo.
Adds 245 Daltons to oligo mass
Production number suffix: -5E
Example 12-04Apr05A-5E
 Back to Index of Modifications
Back to Index of Modifications5'-Cyclooctyne v1, no longer available
5'-Cyclooctyne v1 is a functional group for linking a compound to a Morpholino oligo using copper-free click chemistry.
This structure is in 5'-cyclooctynylated oligos synthesized prior to 12 Aug 2021 and is no longer available: see 5'-Cyclooctyne v3 below for our improved version.
Adds 588 Daltons to oligo mass
Production number suffix: -5C
Example 12-04Apr05A-5C
 Back to Index of Modifications
Back to Index of Modifications5'-Cyclooctyne v2, no longer available
5'-Cyclooctyne v2 is a functional group for linking a compound to a Morpholino oligo using copper-free click chemistry.
This structure is in 5'-cyclooctynylated oligos synthesize from 12 Aug 2021 to 31 Dec 2021 and is no longer available: see 5'-Cyclooctyne v3 below for our improved version.
Adds 606 Daltons to oligo mass
Production number suffix: -5C
Example 03-06Sep21A-5C
 Back to Index of Modifications
Back to Index of Modifications5'-Cyclooctyne v3, no longer available
5'-Cyclooctyne v3 is a functional group for linking a compound to a Morpholino oligo using copper-free click chemistry.
This structure is in 5'-cyclooctynylated oligos synthesized after 31 Dec 2021, no longer available.
Adds 544 Daltons to oligo mass
Production number suffix: -5C
Example 21-10Jan22C-5C
 5'-Cyclooctyne v4, current version
5'-Cyclooctyne v4, current version5'-Cyclooctyne v4 is a functional group for linking a compound to a Morpholino oligo using copper-free click chemistry. This structure is in 5'-cyclooctynylated oligos synthesized after 1 April 2025,current version.
Adds 442 Daltons to oligo mass
Production number suffix: -5C
Example 21-10Jan22C-5C
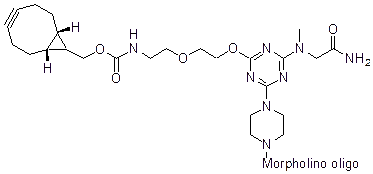
5'-Hydroxy
An inert 5’ end modification
Production number suffix: To Be Determined
Example 21-10Jan22C-5TBD
 Back to Index of Modifications
Back to Index of Modifications